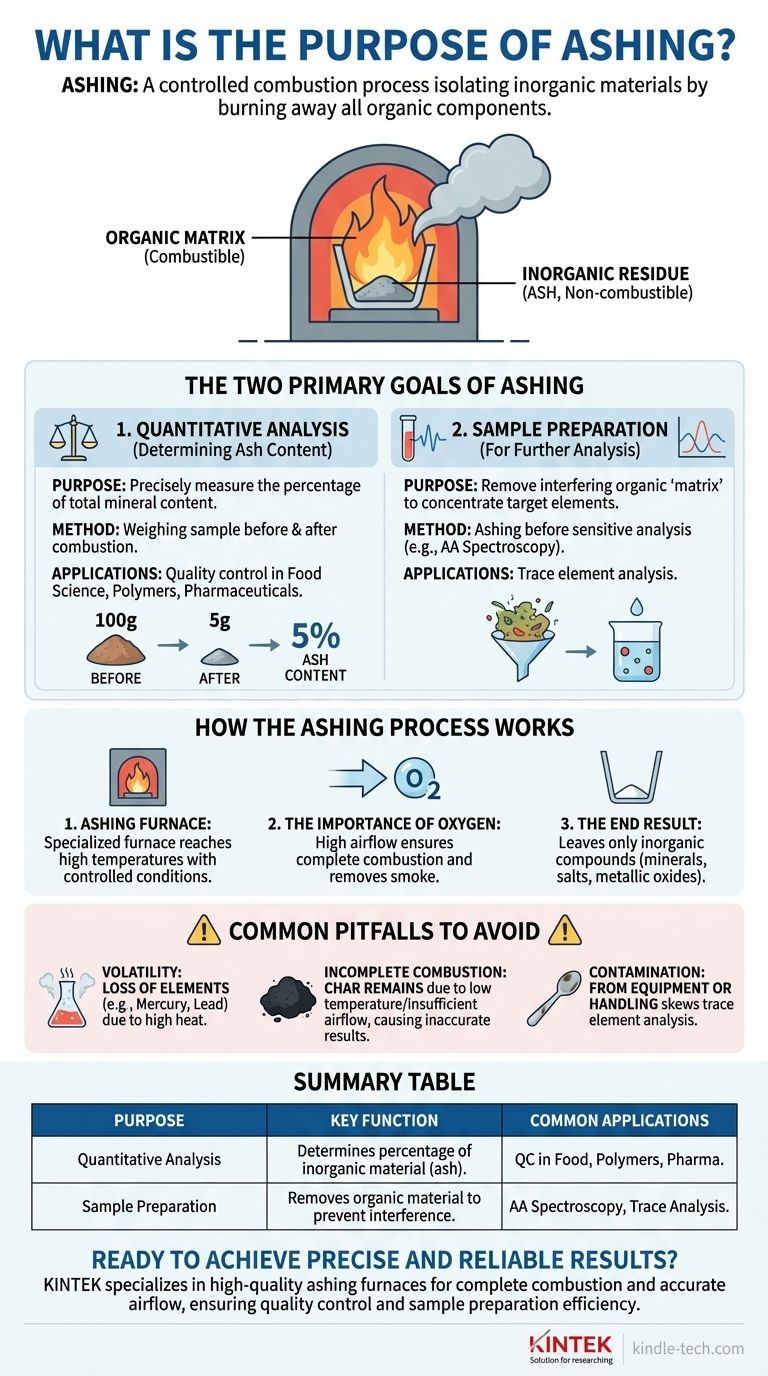
At its core, ashing is a process of controlled combustion. It is an analytical technique that involves heating a sample in the presence of air until all the organic, combustible components burn away. This procedure leaves behind only the inorganic, non-combustible materials, which are collectively known as ash.
The purpose of ashing is not about what is burned away, but about what remains. It is used to either quantify the amount of inorganic material in a sample or to prepare that sample for further elemental analysis by removing interfering organic compounds.

The Two Primary Goals of Ashing
While the method is straightforward combustion, the reason for performing it typically falls into one of two categories: quantifying what's left or purifying the sample for another test.
Quantitative Analysis (Determining Ash Content)
The most common purpose of ashing is to determine the total mineral content within a product.
By measuring the sample's weight before and after complete combustion, you can precisely calculate the percentage of inorganic residue. This is a critical quality control metric in fields like food science, polymer manufacturing, and pharmaceuticals.
Sample Preparation for Further Analysis
Ashing is also a crucial step in preparing samples for more sensitive analytical methods, such as atomic absorption (AA) spectroscopy.
In these cases, the large volume of organic material (the "matrix") can interfere with the instrument's ability to accurately measure the target analyte, such as a specific heavy metal.
By ashing the sample first, you effectively remove this interference, concentrating the inorganic elements of interest for a much cleaner and more accurate measurement.
How the Ashing Process Works
The effectiveness of ashing relies on carefully controlled conditions to ensure complete combustion without losing the target materials.
The Role of the Furnace
The process is performed in a specialized ashing furnace, sometimes called a muffle furnace. This equipment is designed to reach high temperatures while promoting a high level of airflow.
The Importance of Oxygen
Combustion is a chemical reaction with oxygen. The furnace's high airflow design ensures a constant supply of oxygen to the sample, facilitating a rapid and complete burn of all organic components.
This airflow also serves to efficiently remove the smoke and gases created during the process.
The End Result: Inorganic Residue
After the process is complete, the only thing remaining in the crucible is the ash. This residue consists of the inorganic compounds, such as minerals, salts, and metallic oxides, that were present in the original sample.
Common Pitfalls to Avoid
While effective, the ashing process has limitations that are critical to understand for accurate results.
Volatility of Certain Elements
High temperatures can cause certain inorganic or metallic compounds to vaporize and be lost. Elements like mercury, lead, and even some alkali salts can be volatile, which would lead to an underestimation of their presence in the final analysis.
Incomplete Combustion
If the temperature is too low or the airflow is insufficient, the organic material may not burn away completely, leaving behind a carbonized residue known as char. This leads to an inaccurate, artificially high measurement of ash content.
Sample Contamination
For trace element analysis, any contamination from the crucible, the furnace, or handling can significantly skew results. Using impeccably clean equipment is essential for obtaining reliable data.
Making the Right Choice for Your Goal
The application of ashing is directly tied to your analytical objective.
- If your primary focus is quality control or material composition: Use ashing to precisely quantify the percentage of inorganic filler, minerals, or contaminants in your sample.
- If your primary focus is trace element analysis: Use ashing as a sample preparation step to eliminate the organic matrix that could interfere with your spectroscopic measurements.
Ultimately, understanding the purpose of ashing allows you to transform a complex raw sample into a clear, analyzable result.
Summary Table:
| Purpose | Key Function | Common Applications |
|---|---|---|
| Quantitative Analysis | Determines the percentage of inorganic material (ash) in a sample. | Food science, polymer manufacturing, pharmaceuticals (quality control). |
| Sample Preparation | Removes organic material to prevent interference in sensitive analyses. | Preparing samples for atomic absorption (AA) spectroscopy (trace element analysis). |
Ready to achieve precise and reliable results with your ashing process?
KINTEK specializes in high-quality lab equipment, including robust ashing furnaces designed for complete combustion and accurate airflow. Our solutions help laboratories in food science, pharmaceuticals, and materials analysis ensure quality control and prepare samples for sensitive elemental analysis.
Contact us today using the form below to discuss how our equipment can enhance your lab's capabilities and efficiency.
Visual Guide
Related Products
- 1800℃ Muffle Oven Furnace for Laboratory
- 1700℃ Muffle Oven Furnace for Laboratory
- Laboratory Muffle Oven Furnace Bottom Lifting Muffle Furnace
- High Temperature Muffle Oven Furnace for Laboratory Debinding and Pre Sintering
- 1400℃ Muffle Oven Furnace for Laboratory
People Also Ask
- What is the inside material of the muffle furnace? Discover the Refractory Core for High-Temp Precision
- What are 2 advantages of dry ashing? Achieve High-Throughput Sample Analysis with Safety
- What is the process of a muffle furnace? From Electricity to Precision High-Temp Control
- What are the hazards of a muffle furnace? Understanding the Critical Risks for Lab Safety
- At what temperature is it safe to open a muffle furnace? A Guide to Preventing Injury and Equipment Damage



















