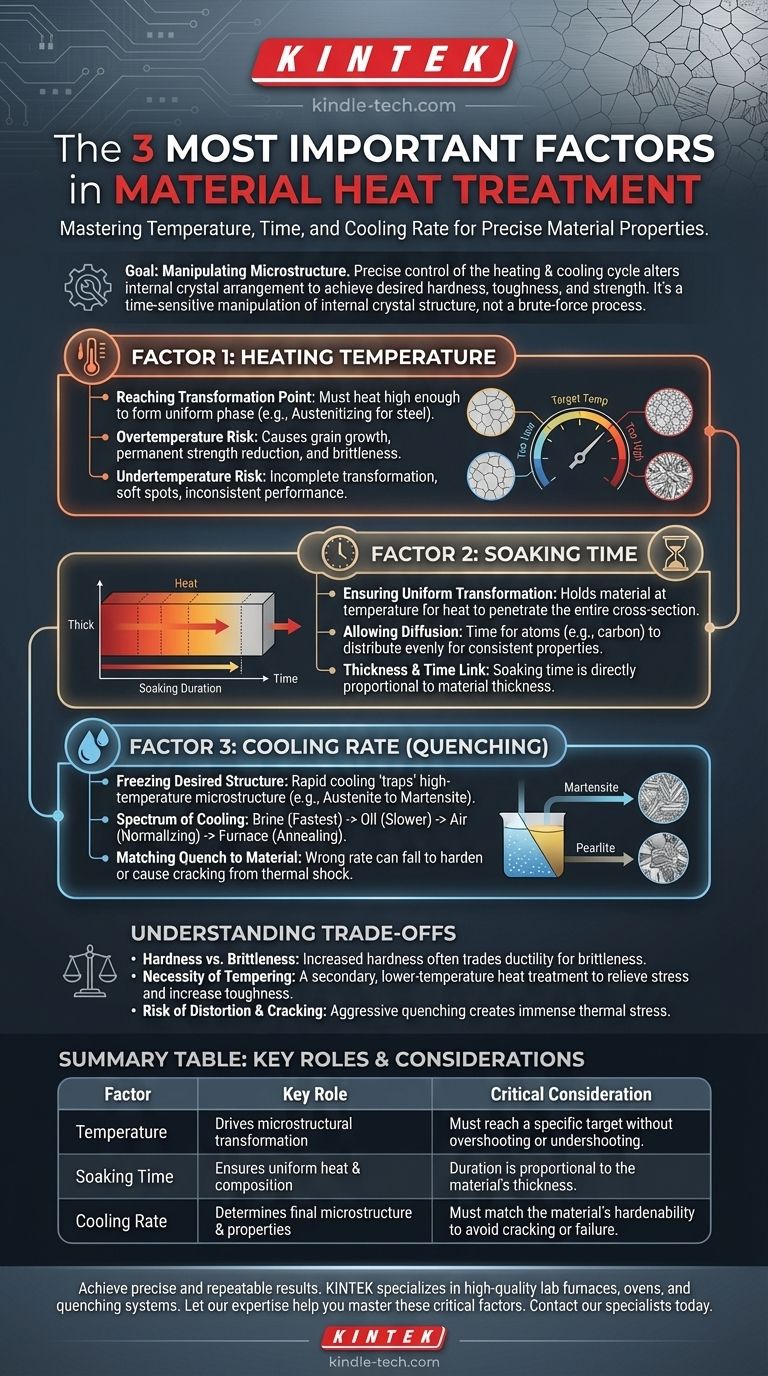
The three most critical factors in material heat treatment are heating temperature, soaking time (the duration at that temperature), and the cooling rate. Mastering the interplay between these three variables is the key to predictably changing a material's physical properties, such as its hardness, toughness, and strength.
Heat treatment is not a brute-force process of heating and cooling. It is a precise and time-sensitive manipulation of a material’s internal crystal structure, where temperature, time, and cooling rate are the primary levers for achieving a specific, desired outcome.

The Foundation: Why Heat Treatment Works
The Goal: Manipulating the Microstructure
All heat treatment processes are designed to alter a material's microstructure, which is the arrangement of its internal crystals.
By controlling the heating and cooling cycle, we can change the size, shape, and type of these crystal structures to produce specific mechanical properties.
From Soft to Hard
In steels, for example, heating above a critical point transforms the structure into a phase called austenite, where carbon is dissolved uniformly. The way this austenite is cooled determines the final properties of the steel.
Factor 1: The Critical Role of Temperature
Reaching the Transformation Point
The first step is always heating the material to a specific target temperature. This temperature must be high enough to force the existing microstructure to transform into a new, uniform phase (like the austenitizing temperature for steel).
The Problem of Overtemperature
Exceeding the target temperature is dangerous. It can cause the material's crystal grains to grow too large, which permanently reduces strength and makes the material brittle, even after a successful quench.
The Problem of Undertemperature
Failing to reach the target temperature results in an incomplete transformation. This leaves soft spots and inconsistencies in the final part, leading to unreliable performance and premature failure.
Factor 2: The Importance of Time (Soaking)
Ensuring Uniform Transformation
Once at temperature, the material must be held there for a specific duration, known as soaking time. This allows heat to penetrate the entire cross-section of the part, ensuring the core reaches the same temperature as the surface.
Allowing for Diffusion
Soaking also provides the necessary time for atoms, like carbon in steel, to diffuse and distribute evenly throughout the crystal structure. This uniformity is essential for a consistent transformation during cooling.
The Link Between Thickness and Time
The required soaking time is directly proportional to the material's thickness. Thicker components require significantly longer soaking times than thinner ones to achieve a uniform internal temperature and composition.
Factor 3: The Decisive Factor of Cooling Rate (Quenching)
"Freezing" a Desired Structure
The rate at which a material is cooled from its target temperature is often the most decisive factor. A rapid cooling process, or quench, is designed to "trap" the high-temperature microstructure before it can transform into softer phases.
For steel, this rapid cooling forces the austenite to become martensite, an extremely hard and brittle crystal structure that is the basis for high-strength components.
The Spectrum of Cooling
Cooling rates exist on a wide spectrum. Quenching in brine or water is extremely fast, while quenching in oil is slower. Even slower methods include cooling in open air (normalizing) or leaving the part to cool slowly inside the furnace (annealing).
Matching the Quench to the Material
Different materials have different hardenability, which is the ability to form martensite. Some alloys require an extremely fast quench, while others can be hardened with a much slower one. Using the wrong quench can either fail to harden the part or, worse, cause it to crack from thermal shock.
Understanding the Trade-offs
Hardness vs. Brittleness
The core trade-off in hardening is that you almost always exchange ductility for hardness. The hard martensitic structure created by quenching is also very brittle and filled with internal stress, making it unsuitable for most applications without further processing.
The Necessity of Tempering
Because of this brittleness, a hardened part is almost always subjected to a second, lower-temperature heat treatment called tempering. This process relieves internal stresses and trades a small amount of hardness for a significant and critical increase in toughness.
Risk of Distortion and Cracking
Aggressive quenching is a violent process that creates immense thermal stress. This can cause parts to warp, distort, or even crack, especially if they have complex geometries with both thick and thin sections.
Making the Right Choice for Your Goal
- If your primary focus is maximum hardness: You need precise control of the peak temperature, a sufficient soak time for the part's thickness, and the fastest cooling rate the specific alloy can withstand without cracking.
- If your primary focus is toughness and durability: You will need to follow a hardening quench with a carefully controlled tempering process, or use a slower cooling method like normalizing or annealing from the start.
- If your primary focus is consistency and reliability: You must prioritize absolute control and repeatability across all three factors—temperature uniformity in the furnace, precise timing for soaking, and a managed, consistent quenching environment.
Ultimately, controlling these three fundamental variables provides direct control over the final properties and performance of the material.
Summary Table:
| Factor | Key Role | Critical Consideration |
|---|---|---|
| Temperature | Drives microstructural transformation | Must reach a specific target without overshooting or undershooting |
| Soaking Time | Ensures uniform heat and composition | Duration is proportional to the material's thickness |
| Cooling Rate | Determines final microstructure and properties | Must match the material's hardenability to avoid cracking or failure |
Achieve precise and repeatable results in your lab. The success of your heat treatment processes hinges on exact control over temperature, time, and cooling. KINTEK specializes in high-quality lab furnaces, ovens, and quenching systems designed to deliver the reliability and consistency your laboratory needs.
Let our expertise in lab equipment help you master these three critical factors. Contact our specialists today to discuss the perfect thermal processing solution for your specific materials and application goals.
Visual Guide
Related Products
- Vacuum Heat Treat Furnace with Ceramic Fiber Liner
- Molybdenum Vacuum Heat Treat Furnace
- 2200 ℃ Tungsten Vacuum Heat Treat and Sintering Furnace
- 2200 ℃ Graphite Vacuum Heat Treat Furnace
- Vacuum Heat Treat Sintering Brazing Furnace
People Also Ask
- Do cannabinoids evaporate? How to Preserve Potency and Prevent Degradation
- Is heat capacity the same for the same material? Understanding Intrinsic vs. Extrinsic Properties
- What is the FTIR used to measure? Identify Chemical Bonds & Molecular Structure
- Is it fitting the mould or mold? A Guide to Correct Spelling by Region
- Are biofuels cheaper to produce than fossil fuels? The True Cost of Green Energy Explained
- What are the advantages of using specialized supports in out-of-pack aluminizing? Achieve a Pristine Surface Finish
- What are the 4 main types of casting? A Guide to Choosing the Right Process
- How does heat treatment affect metal microstructure? Unlock Desired Hardness, Toughness, and Ductility



















