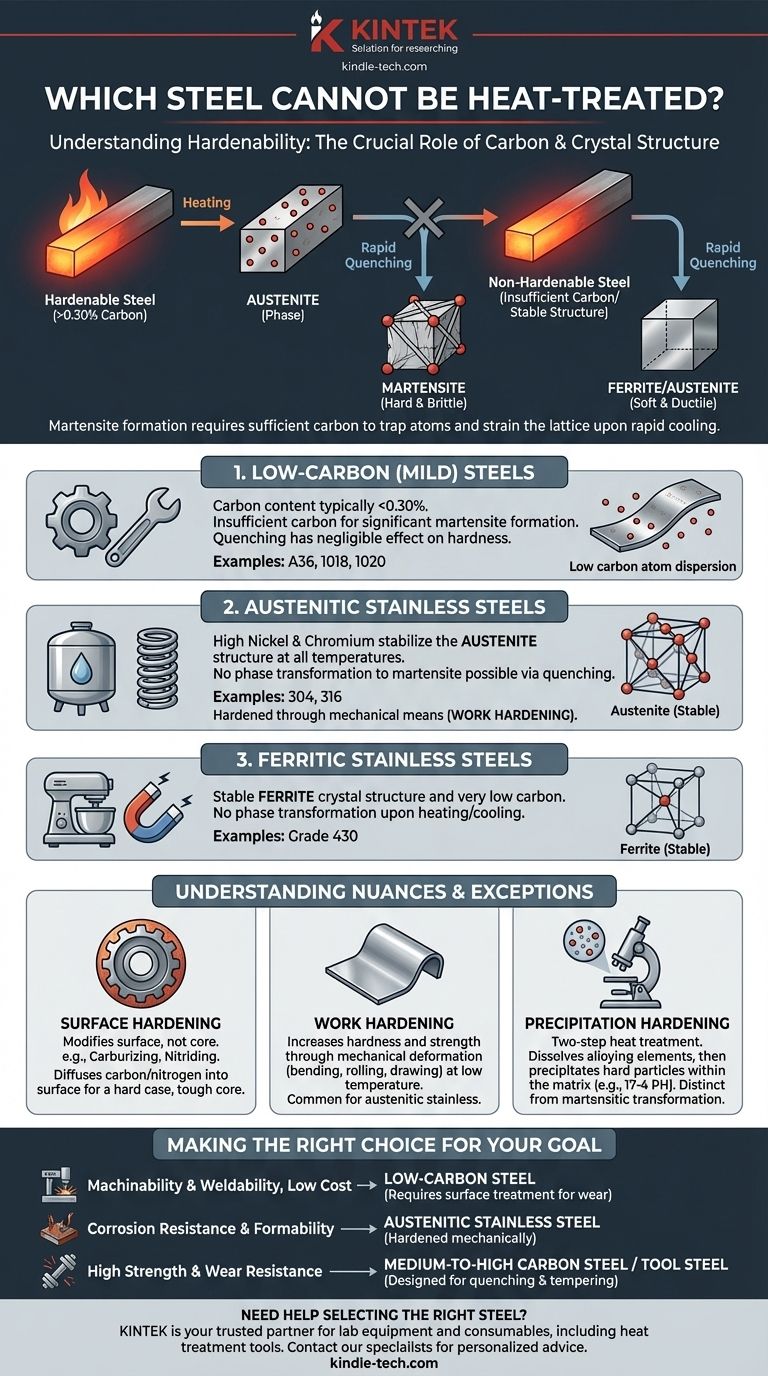
Fundamentally, the ability of a steel to be hardened by heat treatment is determined by its carbon content. Steels with insufficient carbon, or those with crystal structures stabilized by other alloying elements, cannot be meaningfully hardened through the common quenching and tempering process. The primary categories of non-hardenable steels are low-carbon steels, austenitic stainless steels, and ferritic stainless steels.
The capacity for a steel to harden is not an inherent property of all steel; it is a direct result of having enough carbon to form a hard, brittle microstructure called martensite upon rapid cooling. Without sufficient carbon, this transformation is impossible.

The Principle: Why Carbon Governs Hardenability
To understand why some steels can't be heat-treated, we must first understand how hardening works. It isn't the heat itself that hardens the steel, but the structural transformation it enables.
The Role of Martensite Formation
The conventional hardening process involves heating steel until its crystal structure changes to a phase called austenite. In this state, carbon atoms dissolve into the iron lattice.
If the steel is then cooled rapidly (quenched), the carbon atoms become trapped. This forces the iron lattice into a new, highly strained, and very hard structure known as martensite.
The Minimum Carbon Threshold
This transformation to martensite simply cannot occur without a critical amount of carbon. Generally, a steel must have at least 0.30% carbon to exhibit significant hardening.
Steels below this threshold do not have enough dissolved carbon to create the internal strain needed to form a substantial amount of martensite.
Categories of Non-Hardenable Steels
Based on this principle, we can identify several major classes of steel that are not suitable for conventional hardening.
Low-Carbon (Mild) Steels
This is the most common category. Low-carbon steels, often called mild steels, are defined by their low carbon content, typically below 0.30%.
Examples like A36 structural steel, 1018, and 1020 steel are prized for their ductility, weldability, and low cost, but they lack the carbon required for through-hardening. Quenching them has a negligible effect on their hardness.
Austenitic Stainless Steels
This group, which includes the extremely common 304 and 316 grades, has a different reason for being non-hardenable. Their chemistry, high in nickel and chromium, makes their crystal structure austenitic at all temperatures, from cryogenic to melting point.
Since they never leave the austenite phase, the transformation to martensite cannot be triggered by quenching. These steels are non-magnetic and are hardened through mechanical means (work hardening), not heat treatment.
Ferritic Stainless Steels
Similar to austenitic grades, ferritic stainless steels like grade 430 have a stable crystal structure. Theirs is called ferrite, which is the same phase pure iron exists in at room temperature.
These steels have very low carbon content and do not undergo the necessary phase transformation upon heating, making them non-hardenable by heat treatment.
Understanding the Nuances and Exceptions
The statement "cannot be heat-treated" comes with important caveats. While these steels cannot be through-hardened by quenching, other thermal processes can modify their properties.
Surface Hardening: Changing the Surface, Not the Core
Even a low-carbon steel can be given a hard, wear-resistant surface. Processes like carburizing or nitriding are thermochemical treatments that diffuse carbon or nitrogen atoms into the surface of the steel.
This creates a thin, high-carbon (or high-nitrogen) "case" on the part. This case can then be quenched to form martensite, resulting in a hard exterior while the ductile, low-carbon core remains soft and tough.
Work Hardening: A Mechanical Alternative
As mentioned with austenitic stainless steels, work hardening (or strain hardening) is a primary method for increasing the hardness and strength of non-hardenable alloys.
Bending, rolling, or drawing the metal at a low temperature introduces dislocations in the crystal structure, making it more resistant to further deformation. This is how a soft stainless steel sheet becomes a strong spring or a durable kitchen sink.
Precipitation Hardening: A Different Thermal Process
Some specialty stainless steels, such as 17-4 PH, are hardened by a completely different mechanism. This is a two-step heat treatment. First, a solution treatment dissolves alloying elements, and then a lower-temperature "aging" treatment causes microscopic, hard particles to precipitate within the metal matrix.
While this is a form of heat treatment, it is distinct from the martensitic transformation that people usually refer to when discussing the hardening of carbon and alloy steels.
Making the Right Choice for Your Goal
Selecting the correct material requires understanding these distinctions and matching the steel's properties to your application's demands.
- If your primary focus is machinability and weldability at low cost: Low-carbon steel is the default choice, but understand it won't hold an edge or resist wear without a secondary surface treatment.
- If your primary focus is corrosion resistance and formability: Austenitic stainless steel is ideal, but know that its final hardness is determined by mechanical work, not thermal hardening.
- If your primary focus is high strength and wear resistance: You must select a medium-to-high carbon steel or a tool steel specifically designed for hardening via quenching and tempering.
Understanding the relationship between carbon, crystal structure, and heat treatment empowers you to select the precise material your application demands.
Summary Table:
| Steel Category | Examples | Primary Reason for Non-Hardenability |
|---|---|---|
| Low-Carbon (Mild) Steels | A36, 1018, 1020 | Carbon content below ~0.30%, insufficient for martensite formation |
| Austenitic Stainless Steels | 304, 316 | Stable austenitic crystal structure at all temperatures |
| Ferritic Stainless Steels | 430 | Stable ferritic crystal structure, very low carbon content |
Need Help Selecting the Right Steel for Your Application?
Choosing the correct material is critical to your project's success. Whether you need a hardenable tool steel for wear resistance or a non-hardenable stainless steel for superior corrosion resistance, KINTEK is your trusted partner.
We specialize in providing high-quality lab equipment and consumables, including furnaces and tools essential for heat treatment processes. Our experts can help you select the perfect steel for your specific needs, ensuring optimal performance and cost-efficiency.
Let us help you achieve your material goals. Contact our specialists today for personalized advice and solutions tailored to your laboratory's requirements.
Visual Guide
Related Products
- Vacuum Heat Treat Furnace with Ceramic Fiber Liner
- Molybdenum Vacuum Heat Treat Furnace
- 2200 ℃ Tungsten Vacuum Heat Treat and Sintering Furnace
- 1700℃ Laboratory High Temperature Tube Furnace with Alumina Tube
- Graphite Vacuum Furnace High Thermal Conductivity Film Graphitization Furnace
People Also Ask
- What is the process of a vacuum furnace? Achieve Purity and Precision in High-Temp Processing
- What are the advantages of vacuum hardening? Achieve Superior Precision and Cleanliness for Critical Components
- What is the structure of a vacuum furnace? A Guide to Its Core Components & Functions
- What is the maximum temperature in a vacuum furnace? It Depends on Your Materials and Process Needs
- What is vacuum heat treatment process? Achieve Superior Control, Cleanliness, and Quality



















