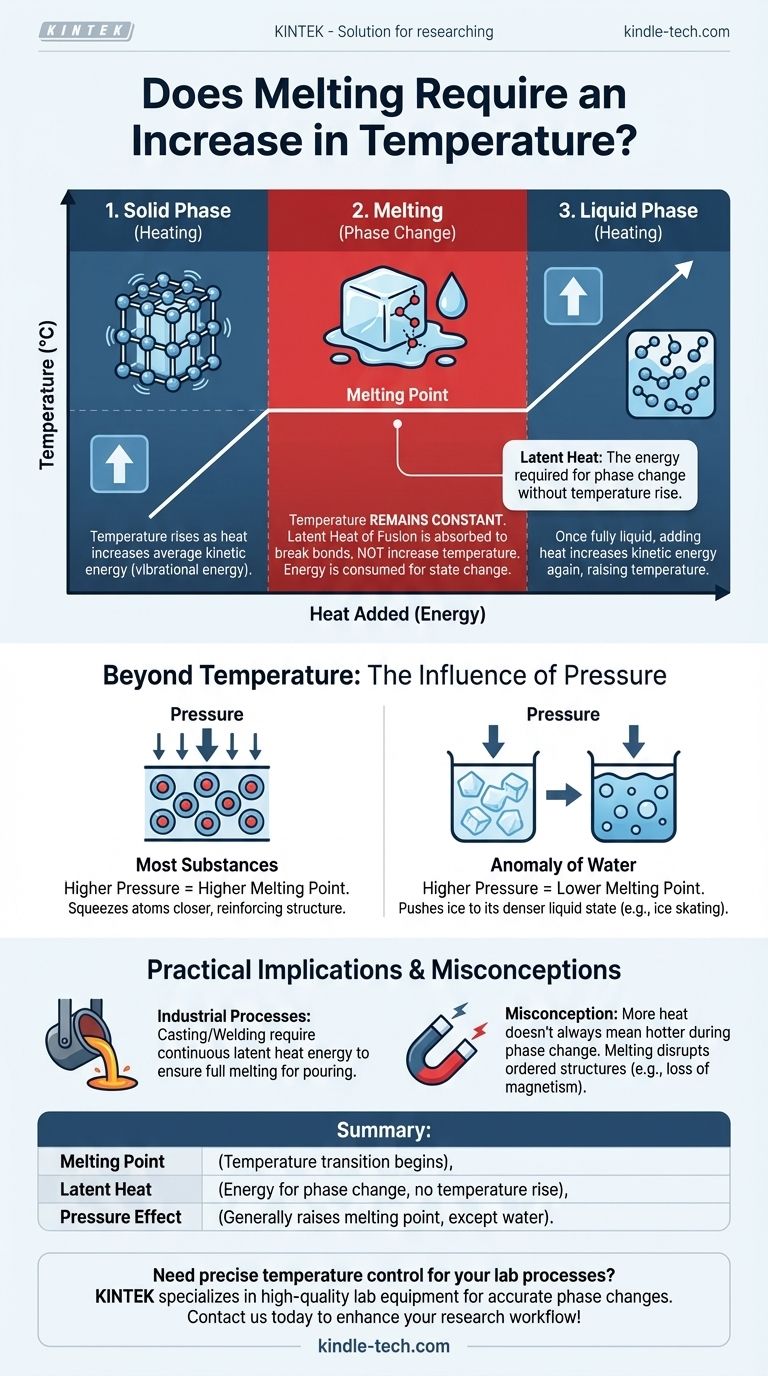
The relationship between heat, temperature, and melting is often misunderstood. To melt a solid, you must add energy in the form of heat. This process first raises the substance's temperature until it reaches its specific melting point. However, during the actual transition from solid to liquid, the temperature remains constant even as more heat is supplied.
While a substance must first be heated to its melting point, the process of melting itself occurs at a constant temperature. The added energy, known as latent heat, is used to break the bonds of the solid structure, not to increase the substance's overall temperature.

The Physics of a Phase Change
To grasp why melting occurs at a constant temperature, we must first distinguish between heat and temperature. They are related, but not the same.
What is Temperature?
Temperature is a measure of the average kinetic energy of the atoms or molecules within a substance. In a solid, these atoms are locked into a fixed structure, like a crystal lattice, but they are constantly vibrating. As you add heat, you increase this vibrational energy, which we measure as a rise in temperature.
Reaching the Melting Point
As you continue to add heat, the atoms vibrate more and more violently. Eventually, they reach an energy level where the vibrations are strong enough to begin breaking the rigid bonds holding them in their fixed positions. This specific temperature is the melting point.
The Role of Latent Heat
At the melting point, a critical change occurs. Any additional heat energy you supply—known as the latent heat of fusion—is now used exclusively to break the remaining bonds of the solid structure. It is not used to make the molecules vibrate faster.
Because the energy is being consumed to change the substance's state (from solid to liquid) rather than increase its kinetic energy, the temperature remains perfectly constant until the entire solid has turned into a liquid.
The Liquid State
Once the last of the solid has melted, the substance is fully in a liquid state. From this point on, any further heat you add will once again increase the kinetic energy of the molecules, causing the liquid's temperature to rise.
Beyond Temperature: The Influence of Pressure
Temperature is not the only factor that governs melting. Pressure plays a crucial, and sometimes counterintuitive, role.
How Pressure Affects Melting
For most substances, increasing the external pressure raises the melting point. High pressure physically squeezes the atoms closer together, reinforcing the solid structure. This means more energy (and thus a higher temperature) is required to break the bonds and allow the substance to melt.
The Anomaly of Water
Water is a notable exception. The solid form of water (ice) is less dense than its liquid form. Increasing pressure on ice pushes it toward its denser state—liquid water. This means that under high pressure, ice can melt at a temperature below its standard melting point of 0°C (32°F). This principle contributes to how the blade of an ice skate glides over ice.
Practical Implications and Misconceptions
Understanding this process is not just academic; it has direct consequences in science and engineering.
Misconception: More Heat Always Means Hotter
The most common misconception is that continuously adding heat will always make something hotter. During a phase change, like melting or boiling, this is incorrect. The energy input is performing the work of changing the material's state, not increasing its temperature.
Why This Matters in Practice
In industrial processes like casting or welding metals, this principle is critical. A furnace must not only bring a metal to its melting point but also supply a significant and continuous amount of additional energy (the latent heat) to ensure the entire mass becomes fully liquid for pouring. Similarly, melting disrupts the ordered atomic structure of materials. This is why heating a magnetized piece of steel past a certain point (its Curie temperature, which is related to but distinct from its melting point) will cause it to lose its magnetism, as the organized magnetic domains are broken apart.
Making the Right Choice for Your Goal
Your application determines which part of this process is most important to you.
- If your primary focus is understanding basic physics: Remember that temperature is constant during a phase change; the added energy is called latent heat and is used to change the state.
- If your primary focus is materials science or engineering: Recognize that fully melting a substance requires a precise and continuous energy input, even after it has reached its melting temperature.
- If you are considering environmental effects: Be aware that pressure can significantly alter a substance's melting point, raising it for most materials but lowering it for an important exception like water.
Understanding this distinction between temperature and heat energy is fundamental to controlling and predicting the behavior of any material.
Summary Table:
| Concept | Description |
|---|---|
| Melting Point | Temperature where a solid begins to transition to liquid. |
| Latent Heat of Fusion | Energy absorbed during melting without temperature change. |
| Pressure Effect | High pressure raises melting point (except for water, which lowers it). |
| Practical Application | Critical for industrial processes like metal casting and welding. |
Need precise temperature control for your lab processes? KINTEK specializes in high-quality lab equipment, including furnaces and heating systems, designed to handle phase changes like melting with accuracy. Whether you're working with metals, polymers, or other materials, our solutions ensure efficient energy management and consistent results. Contact us today to explore how our lab equipment can enhance your research or production workflow!
Visual Guide
Related Products
- Laboratory Muffle Oven Furnace Bottom Lifting Muffle Furnace
- 1800℃ Muffle Oven Furnace for Laboratory
- 1700℃ Muffle Oven Furnace for Laboratory
- 1400℃ Muffle Oven Furnace for Laboratory
- 1400℃ Laboratory High Temperature Tube Furnace with Alumina Tube
People Also Ask
- Why is an ultrasonic homogenizer used for LNMO samples? Ensure Accurate Particle Size Distribution Analysis
- What is sintered glass used for? Achieve Pure Filtration & Gas Diffusion in Your Lab
- What is the effect of sputtering pressure? Master Atomic Energy for Superior Thin Films
- Which institutions have adjusted their ULT freezer set points to -70°C? Save Energy Without Risking Samples
- What is the role of a high-pressure homogenizer in PHA extraction? Optimize Your Bio-Material Recovery Process
- What is the difference between microwave and conventional pyrolysis? A Guide to Heating Mechanisms & Efficiency
- What is sputtering yield? Master the Key to Efficient Thin Film Deposition
- Which type of evaporator is used in chemical industry? Choose the Right Evaporator for Your Process



















