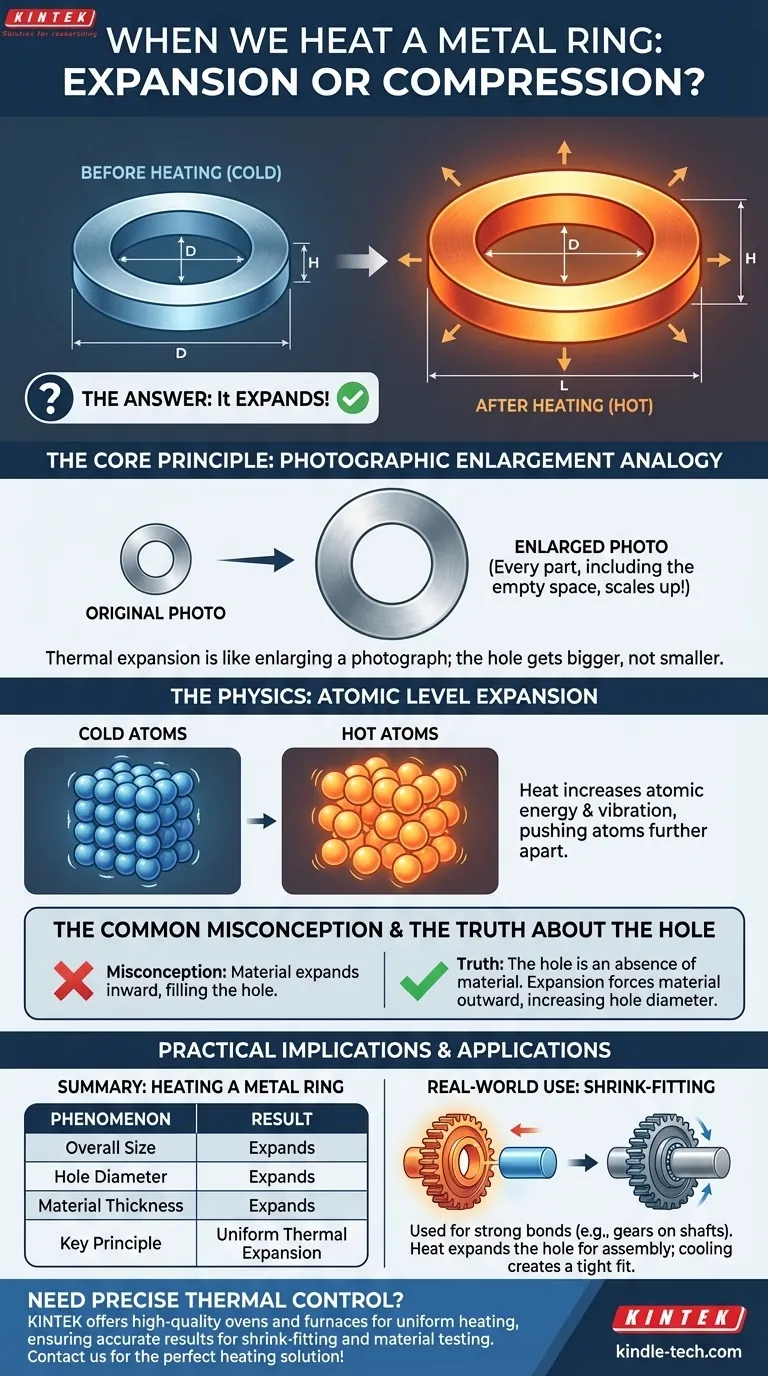
To be direct, when you heat a metal ring, it expands. Every dimension of the ring increases, including its thickness, its overall diameter, and perhaps most counter-intuitively, the diameter of the hole in the center. The hole gets bigger, not smaller.
The core principle to understand is that thermal expansion is not like the material swelling inward to fill a void. Instead, think of it as a photographic enlargement: every single part of the object, including the empty space it defines, scales up proportionally.

The Physics of Thermal Expansion
How It Works at an Atomic Level
When you apply heat to a metal, you are transferring energy to its atoms. This increased energy causes the atoms to vibrate more vigorously.
As they vibrate, they push against their neighbors, increasing the average distance between them. This separation, scaled up across trillions of atoms, results in a measurable expansion of the object in all directions.
The Uniform Expansion Rule
This expansion occurs uniformly throughout the material, provided the heat is applied evenly.
It doesn't just happen on the outer edges. The material expands outward, inward, and in every dimension simultaneously.
The Core Question: What Happens to the Hole?
This is the concept that trips most people up. It's easy to imagine the metal expanding and "squeezing" the hole, making it smaller. However, the opposite is true.
The Common Misconception
The error in thinking is viewing the hole as a separate entity that the material can expand into.
The hole is not an object; it is an absence of material. The expansion is dictated by the behavior of the material that is present.
The Correct Analogy: A Photographic Enlargement
Imagine you have a photograph of the ring. If you use a photocopier to enlarge that photo by 10%, everything in the picture gets 10% bigger.
The outer edge is 10% larger, the thickness of the ring is 10% larger, and the hole in the middle is also 10% larger. The atoms on the inner circumference are pushed away from each other, just like the atoms on the outer circumference, which forces the diameter of the hole to increase.
Key Factors and Practical Implications
Uniform Heating is Crucial
This principle assumes the entire ring is heated to the same temperature.
If you only heat the outside of a very thick ring, the outer part will expand while the cooler inner part does not, creating internal stresses. For most standard rings, however, the heat distributes quickly enough for the effect to be uniform.
The Coefficient of Thermal Expansion
Different materials expand at different rates. This property is known as the coefficient of thermal expansion.
Aluminum, for example, expands significantly more than steel for the same temperature change. This is a critical factor in engineering and design, especially when different metals are joined together.
Real-World Applications
This principle is not just a thought experiment; it's a fundamental technique in mechanical engineering.
This process, known as shrink-fitting or interference fitting, is used to create extremely strong bonds between parts. A gear can be heated to expand its central hole, allowing it to be easily slipped onto a shaft. As it cools, it contracts and grips the shaft with immense pressure.
How This Principle is Used in Practice
Understanding this concept allows you to predict and use the behavior of materials.
- If your primary goal is to fit a shaft into a ring: Heat the ring to expand the hole, allowing for an easy assembly.
- If your primary goal is to separate two stuck metal parts (like a bolt and nut): Heat the outer part (the nut), which will cause it to expand and break its seal with the cooler inner part (the bolt).
- If your primary goal is to design a high-precision assembly: You must account for the different expansion rates of materials that will operate at varying temperatures.
Ultimately, remember that heat makes the atoms in a material move apart, causing the entire object and the spaces it contains to grow.
Summary Table:
| Phenomenon | Result of Heating a Metal Ring |
|---|---|
| Overall Size | Expands |
| Hole Diameter | Expands |
| Material Thickness | Expands |
| Key Principle | Uniform Thermal Expansion |
| Practical Use | Shrink-Fitting for Assembly |
Need precise thermal control for your material testing or assembly processes?
At KINTEK, we specialize in high-quality lab equipment, including ovens and furnaces that provide the uniform heating essential for reliable thermal expansion experiments and industrial applications like shrink-fitting. Our solutions help engineers and researchers achieve accurate, repeatable results.
Contact us today to find the perfect heating solution for your laboratory needs!
Visual Guide
Related Products
- Laboratory Muffle Oven Furnace Bottom Lifting Muffle Furnace
- 1800℃ Muffle Oven Furnace for Laboratory
- 1400℃ Muffle Oven Furnace for Laboratory
- 1700℃ Muffle Oven Furnace for Laboratory
- 1400℃ Laboratory High Temperature Tube Furnace with Alumina Tube
People Also Ask
- What is the use of muffle furnace in food laboratory? Essential for Accurate Nutritional Analysis & Quality Control
- What temperature should a furnace run at? From Home Comfort to Industrial Processes
- What construction features contribute to the practicality and reliability of a muffle furnace? Key Design Elements for Lab Success
- What is the operating temperature of the muffle furnace? Find Your Ideal Range for Lab Success
- How does a high-temperature muffle furnace work? Achieve Contaminant-Free, Uniform Heating



















