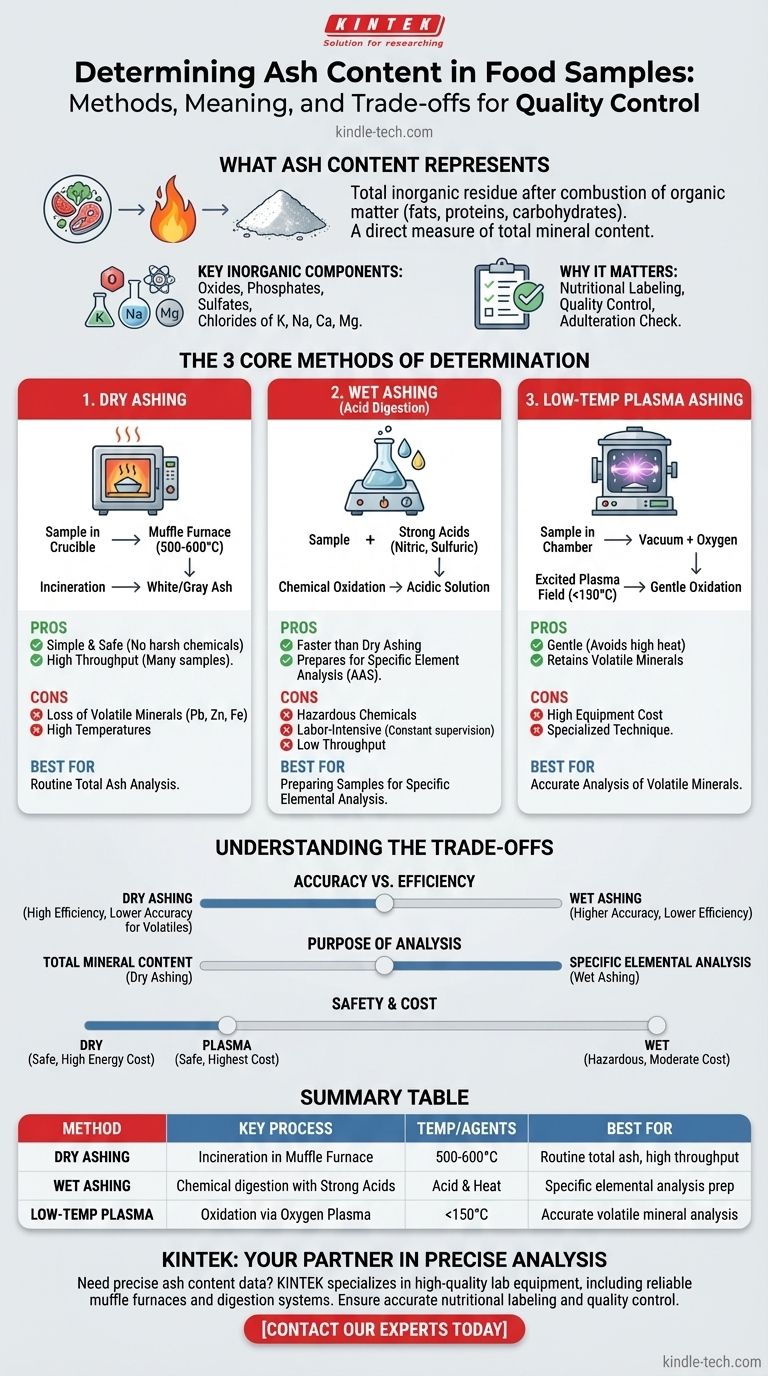
To determine the ash content of a food sample, the sample is completely incinerated at a high temperature to burn away all organic matter, such as fats, proteins, and carbohydrates. The remaining noncombustible, inorganic residue is the ash, which is then cooled and weighed. The primary laboratory techniques for this are dry ashing, wet ashing, and low-temperature plasma ashing.
Ash content measurement is a fundamental analysis in food science that quantifies the total amount of inorganic minerals in a product. The choice of method is not arbitrary; it depends entirely on whether you need a simple total mineral value or are preparing the sample for a more sensitive analysis of specific elements.

What Ash Content Actually Represents
A Proxy for Total Mineral Content
The "ash" is the inorganic residue left after the complete combustion of organic matter. Its weight provides a direct measure of the total mineral content within a food sample.
Key Inorganic Components
This residue is primarily composed of the oxides, phosphates, sulfates, and chlorides of essential minerals. Common elements include potassium, sodium, calcium, and magnesium.
Why It Matters in Food Quality
Measuring ash is a critical quality control parameter. It is used for nutritional labeling, checking for the addition of inorganic substances (adulteration), and as an indicator of the quality of certain food ingredients, like flour or spices.
The Core Methods of Ash Determination
Method 1: Dry Ashing
This is the most common method. The sample is placed in a crucible and heated in a muffle furnace at very high temperatures (typically 500-600°C) until only a white or gray ash remains.
The primary advantage of dry ashing is its simplicity and safety, as it avoids harsh chemicals. It also allows for many samples to be processed simultaneously.
However, the high temperatures can cause some volatile minerals, such as lead, zinc, and iron, to be lost, potentially leading to inaccurate results for those specific elements.
Method 2: Wet Ashing (Acid Digestion)
In wet ashing, the sample is broken down using strong acids (like nitric acid and sulfuric acid) and heat. This process chemically oxidizes the organic matter, leaving the minerals in an acidic solution.
This method is significantly faster than dry ashing and is the preferred technique when preparing a sample for the analysis of specific mineral elements using methods like atomic absorption spectroscopy (AAS).
The main drawbacks are that it requires constant technician supervision, uses hazardous chemicals, and is not suitable for processing large batches of samples at once.
Method 3: Low-Temperature Plasma Ashing
This is a more specialized and gentler technique. A sample is placed in a chamber where a vacuum is created, and oxygen is introduced and excited by an electromagnetic field.
This process creates highly reactive oxygen plasma that oxidizes the organic matter at much lower temperatures (usually below 150°C).
Because it avoids high heat, this method is excellent for accurately measuring volatile minerals that would be lost during dry ashing. Its use is limited by the high cost of the specialized equipment required.
Understanding the Trade-offs
Accuracy vs. Efficiency
Dry ashing is highly efficient for determining total ash content on many samples but risks losing volatile minerals. Wet ashing is more accurate for trace mineral analysis but is far more labor-intensive and has lower throughput.
Purpose of the Analysis
If the goal is simply to measure the total mineral content as a single value for a nutrition panel, dry ashing is perfectly suitable. If the goal is to measure the concentration of a specific heavy metal, wet ashing is necessary to prevent volatilization and prepare the sample for further analysis.
Safety and Cost
Dry ashing is relatively safe but has high energy costs. Wet ashing introduces the handling risks of strong, corrosive acids. Low-temperature ashing is the most expensive method due to the initial equipment investment.
Making the Right Choice for Your Analysis
Selecting the appropriate method is crucial for obtaining meaningful data. Your choice should be dictated by the specific analytical goal.
- If your primary focus is routine quality control for total ash percentage: Dry ashing is the industry standard due to its simplicity, safety, and ability to handle large batches.
- If your primary focus is preparing a sample for specific elemental analysis (e.g., heavy metals): Wet ashing is the superior choice because it avoids high-temperature mineral loss and leaves the minerals in a solution ready for testing.
- If your primary focus is highly accurate analysis of volatile minerals with minimal contamination: Low-temperature plasma ashing is the most precise method, provided the specialized equipment is available.
Ultimately, selecting the correct ashing technique ensures your data accurately reflects the true mineral composition of your food product.
Summary Table:
| Method | Key Process | Temperature / Agents | Best For |
|---|---|---|---|
| Dry Ashing | Incineration in a muffle furnace | 500-600°C | Routine total ash analysis, high sample throughput |
| Wet Ashing | Chemical digestion with strong acids | Acid & Heat | Preparing samples for specific elemental analysis |
| Low-Temperature Plasma Ashing | Oxidation via oxygen plasma | <150°C | Accurate analysis of volatile minerals |
Need precise ash content data for your food products? The right equipment is critical for accurate results. KINTEK specializes in high-quality lab equipment, including reliable muffle furnaces for dry ashing and digestion systems for wet ashing. Our solutions help food laboratories ensure accurate nutritional labeling and quality control. Contact our experts today to find the perfect ashing solution for your lab's needs!
Visual Guide
Related Products
- 1800℃ Muffle Oven Furnace for Laboratory
- 1700℃ Muffle Oven Furnace for Laboratory
- High Temperature Muffle Oven Furnace for Laboratory Debinding and Pre Sintering
- Laboratory Muffle Oven Furnace Bottom Lifting Muffle Furnace
- 1400℃ Muffle Oven Furnace for Laboratory
People Also Ask
- Why must a high-temperature furnace be used after diffusion chromizing? Restoring Core Strength and Plasticity
- What is the function of a high-temperature furnace in rare earth oxide production? Achieve High-Purity Material Stability
- For what purpose is a programmed temperature heat treatment furnace used when testing MPCF/Al composites? Space Testing
- What are the primary functions of a muffle furnace for Inconel 718? Achieve Uniform Annealing and Stress Relief
- What is the function of a blast drying oven in A356-SiCp composite powder preparation? Ensure Defect-Free Sintering
- What are the uses of muffle furnace in pharmaceutical industry? Essential for Drug Purity & Safety
- What is the temperature of a sintering oven? Master the Key to Perfect Material Properties
- What is the temperature of a microwave sintering furnace? Achieve Rapid, Uniform Heating Up to 1750°C



















